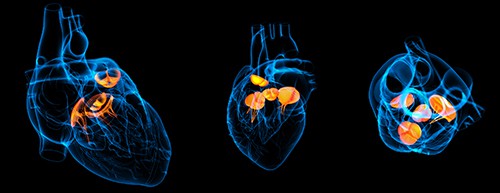
On February 1, Edwards EVOQUE Tricuspid Valve Replacement System was approved by the FDA. The system treats patients with tricuspid regurgitation (TR). It’s the first transcatheter valve replacement therapy to receive regulatory approval to treat TR. This is the first FDA approved transcatheter replacement device for use in the tricuspid position.
Recommended courses: Heart Failure Update: What’s New in the Latest Guidelines
Some people with mild TR don’t experience symptoms. However, for patients with severe cases, their symptoms can drastically decrease their quality of life. Symptoms can include:
- Fatigue
- Irregular heart rhythms (heart dysrhythmias)
- Pulsing in the neck
- Shortness of breath with activity
- Abdomen, leg or neck vein swelling
The EVOQUE system is intended to treat patients who are approved for tricuspid valve replacement. Susheel Kodali, MD, director, Structural Heart and Valve Center at Columbia University Irving Medical Center/New York-Presbyterian Hospital and TRISCEND II Study Principal Investigator, says it can replace the native tricuspid valve, and virtually eliminate TR in a wide range of patients.
RESOURCES