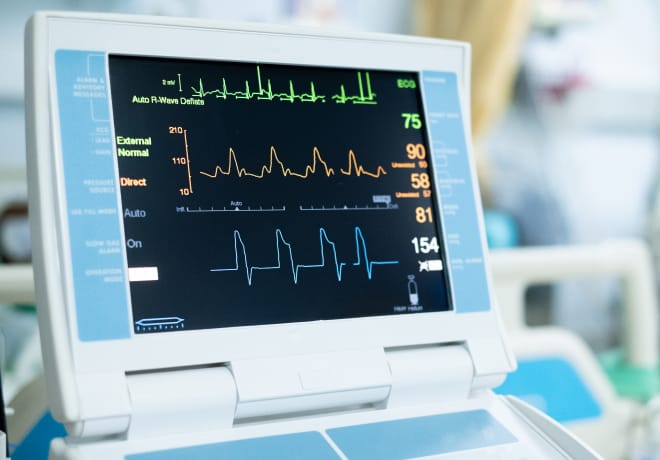
The FDA has qualified the Apple Watch Atrial Fibrillation History Feature as part of its Medical Device Development Tools (MDDT) program. This decision marks the Apple Watch feature as the first digital health technology to gain such recognition, setting a new precedent in the utilization of wearable technology within clinical studies.
Recommended Course: Cardiac Rhythms: The Clinicians Approach to Accurate Interpretation
A non-invasive method for estimating atrial fibrillation
Designed to offer a non-invasive method for estimating atrial fibrillation (AFib) burden, the Apple Watch feature will now play a pivotal role in clinical studies aimed at assessing the safety and effectiveness of cardiac ablation devices. It represents a significant advancement in how researchers can monitor and evaluate AFib burden, enhancing the study of cardiac treatments.
The Apple Watch employs its photoplethysmography (PPG) sensor. This sensor analyzes pulse rate data to identify episodes of irregular heart rhythms akin to AFib. Utilizing green light-emitting diodes (LEDs) and light-sensitive photodiodes, the sensor monitors changes in blood flow through the wearer’s wrist. It also detects heartbeats and allows for the determination of time spent in AFib over the previous week.
Recommended Course: Analysis of the 12-Lead ECG: Beyond the Basics
Atrial fibrillation before and after cardiac ablation treatments
This information is crucial for clinical trials. It provides researchers with retrospective weekly estimates of AFib burden before and after cardiac ablation treatments. The feature’s introduction to clinical settings underscores a significant stride toward integrating digital health technologies. It contributes to a more comprehensive monitoring and understanding of heart health.
The Apple AFib History Feature’s qualification as a biomarker test for evaluating AFib burden solidifies its role as an essential tool in comparative analysis during cardiac studies. However, it’s important to note that the feature aids in secondary effectiveness endpoints. It does not replace primary endpoint findings in determining the safety and efficacy of cardiac ablation devices.
This innovative integration of digital health technology within clinical research frameworks emphasizes the growing importance and reliability of wearable devices in advancing medical science and patient care.